Staying Young and Extending Life By Resetting Blood Proteins
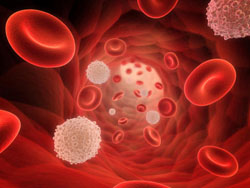
A number of studies have been done where older animals were given blood from younger animals to find out if that would increase life span. It did and also improved the health of the older animals. In a new study, scientists are trying to reverse aging in older humans by filtering bad proteins from their blood and return it to a more youthful state. This comes after a study on mice showed that the procedure had some promise. For instance there was evidence that the blood cells from the younger mice produced muscle repair in the older mice. Now researchers from California are trying to replicate the results in older people in a clinical trial. The aim of this radical approach is to alter levels of bad proteins in the blood of older people. These proteins are believed to be responsible for hampering the growth of healthy tissue. This could help in the prevention of age-related disease and possibly slow down the aging process. The study was published in the journal Nature Communications.
This is part of what we are doing with Stem Cell 100 and Stem Cell 100+. They contain compounds for instance that up regulate longevity genes and help the blood and tissues to a younger state. Of course this occurs over time.
The animal study was co-funded by Calico, which is a life extension company owned by Google. The experiment was reversed on mice with old blood infused into young mice. The results showed a reduction in new liver and brain cells in the young mice and impaired performance in strength. This gave more credibility to the original experiment. In the human trial, older blood will pass through a machine that will try to reset proteins to a healthier level in the hope that body tissues will be properly maintained thus slowing down aging.
This new study is one of many that show key molecules in the blood can alter the pace of aging in body tissue. When these proteins are at low levels the body is healthy, but as we get older these protein levels can change. The team is now considering a more practical approach to control the levels of the proteins without blood transfusions. According to one scientist, these new treatments could prevent diabetes, Alzheimer’s, and Parkinson?s disease.
Another scientist Tony Wyss-Coray, from Stanford University was not convinced by the study and pointed out that only four pairs of mice were used in the experiment. In past experiments on animals, a procedure called parabiosis was used to swap blood between animals by conjoining them surgically. In this study, scientists did not use surgery but instead transferred blood through a tube and pump controlled by a computer.
The team is now working on devices that filter blood in more advanced ways to reduce high levels of the bad proteins. This will return the proteins to more youthful levels. The key here is to remove the inhibitor molecules and then to return the filtered blood back to the recipient. This medical procedure could result in life extension that could give people an extra three decades of life without any critical illnesses. This advanced treatment could become available within 3 years.
The Berkley team is currently brainstorming for ideas on how to normalize the levels of one particular protein considered to be the inhibitor. They hope clinical trials will start within six months and start producing results within three years. Scientist could be on the verge of transforming our lives by slowing the aging process and stopping age-related diseases.
References: Justin Rebo, Melod Mehdipour, Ranveer Gathwala, Keith Causey, Yan Liu, Michael J. Conboy & Irina M. Conboy. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nature Communications, 2016; doi:10.1038/ncomms13363