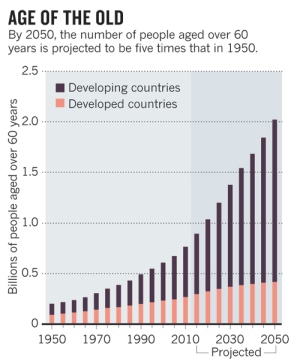
By 2050, the number of people over the age of 80 will triple globally, which could come at great cost to individuals and economies. In a commentary published July 24 in Nature, three experts call for moving forward with preclinical and clinical strategies for people that have been shown to delay aging in animals. In addition to promoting a healthy diet and regular exercise, these strategies include slowing the metabolic and molecular causes of human aging, such as the incremental accumulation of cellular damage that occurs over time.
Unfortunately, medicine focuses almost entirely on fighting chronic diseases in a piecemeal fashion as symptoms develop. Instead, more efforts should be directed to promoting interventions that have the potential to prevent multiple chronic diseases and extend healthy lifespans.
The researchers, at Washington University School of Medicine in St. Louis, Brescia University in Italy, the Buck Institute for Aging and Research and the Longevity Institute at the University of Southern California, write that unfortunately, economic incentives in biomedical research and health care reward treating disease more than promoting good health.
The problems of old age come as a package. More than 70% of people over 65 have two or more chronic conditions. Studies of diet, genes and drugs indicate that delaying one age-related disease probably staves off others. At least a dozen molecular pathways seem to set the pace of physiological ageing.
Researchers have tweaked these pathways to give rodents long and healthy lives. Restricting calorie intake in mice or introducing mutations in nutrient-sensing pathways can extend lifespans by as much as 50%. And these ‘Methuselah mice’ are more likely than controls to die without any apparent diseases. In other words, extending lifespan also seems to increase ‘healthspan’, the time lived without chronic age-related conditions.
Research has highlighted potential benefits from dietary restriction in extending healthy life span. People who eat significantly fewer calories, while still getting optimal nutrition, have ?younger,? more flexible hearts. They also have significantly lower blood pressure, much less inflammation in their bodies and their skeletal muscles function in ways similar to muscles in people who are significantly younger.
These insights have made hardly a dent in human medicine. Biomedicine takes on conditions one at a time. Rather, it should learn to stall incremental cellular damage and changes that eventually yield several infirmities.
The current tools for extending healthy life ? better diets and regular exercise ? are effective. But there is room for improvement, especially in personalizing treatments. Molecular insights from animals should be tested in humans to identify interventions to delay ageing and associated conditions. Together, preclinical and clinical researchers must develop meaningful endpoints for human trials.
Longevity pathways identified in model organisms seem to be conserved in humans and can be manipulated in similar ways. Genetic surveys of centenarians implicate hormonal and metabolic systems. Long-term calorie restriction in humans induces drastic metabolic and molecular changes that resemble those of younger people, notably in inflammatory and nutrient-sensing pathways. Mice engineered to have reduced signalling in these pathways live longer.
Several molecular pathways that increase longevity in animals are affected by approved and experimental drugs. The sirtuin proteins, involved in a similar range of cellular processes, are activated by high concentrations of naturally occurring compounds and extend lifespan in metabolically abnormal obese mice. A plethora of natural and synthetic molecules affect pathways that are shared by ageing and conditions related to ageing.
Diet has similar effects. The drugs rapamycin and metformin mimic changes observed in animals fed calorie and protein-restricted diets. And fasting triggers cellular responses that boost stress resistance, and reduce oxidative damage and inflammation. In rodents, fasting protects against diabetes, cancer, heart disease and neurodegeneration9. There are many anti-ageing interventions that could be considered for clinical trials.
Ignored opportunities
Scientists are not set up to capitalize on these leads to combat the looming ageing crisis. Clinicians do not realize how much is understood about the molecular mechanisms of ageing and its broad effects on diseases. Researchers of all stripes focus too much on easing or reversing the progression of diseases.
The problem is calcified by the funding gap. Budgets for ageing research are small compared to disease-centred research. The Division of Aging Biology in the US National Institute on Aging receives less than 1% of the National Institutes of Health’s budget even though it supports research into the mechanisms underlying most disabilities and chronic diseases. Most grants focus on diseases of specific systems. Most study sections are not set up to evaluate multidisciplinary research on healthspan. The situation is similar in Europe and Japan.
How should we test interventions that extend healthspan? Human data from dietary restriction and genetic-association studies of healthy ageing could help to channel the most promising pathways identified in preclinical studies. Animal studies should be designed to better mimic human ageing. For example, frailty indices are often used in human studies. Comparable indices should be developed for mice.
Suitable endpoints for human trials are needed. Animal work suggests many candidates as potential biomarkers, such as accumulation of molecular damage to DNA, proteins and lipids from oxidative stress. Publicly funded clinical trials could also collect crucial samples of blood, muscle and fat for molecular analysis.
Funding agencies should establish committees of translational scientists to review which markers of biological ageing are most consistent between animals and humans, and prioritize the most practical for further assessment. Chosen biomarkers could be evaluated in clinical studies over a broad age range of patients already being treated with drugs that increase lifespan in animal models. Assessments must also be developed for dietary or other interventions that do not involve drugs.
The most important change must be in mindset. Economic incentives in both biomedical research and health care reward treating diseases more than promoting health. The launch of a few anti-ageing biotech companies such as Calico, created last year by Google, is promising. But public money must be invested in extending healthy lifespan by slowing ageing. Otherwise we will founder in a demographic crisis of increased disability and escalating health-care costs.





