Dr. Centeno is offering stem cell therapies for orthopedic damage with remarkable results. Is this the future of stem cells in the US?
The FDA has yet to approve stem cell therapies for general use in medicine, but that hasn?t stopped doctors in Colorado from providing them anyway. Chris Centeno and John Schultz have boldly formed Regenerative Sciences Inc. in Broomfield, Colorado. RSI provides its patients with the Regenexx procedure, an adult stem cell transplant that uses your own cells (autologous) to treat joint injuries and bone damage. There?s no surgery needed. A needle extracts bone marrow, RSI isolates the stem cells and cultures them in your own blood, and then these cells are injected into the area where they are needed. They?ve treated 348+ patients with 800+ injections and show no signs of slowing down. According to RSI?s own surveys, 89% of their knee patients showed marked improvement, as did 75% of their hip patients! Within months some patients can walk or run in ways they haven?t been able to in years. We?ve seen these kinds of results from stem cell treatments before, but only in horses and dogs. That?s because human stem cell therapies like this one aren?t approved by the FDA. How can Centeno and Schultz flaunt the lack of federal approval? They claim that Regenexx is solely used as a part of their medical practice, only within the state of Colorado, and as such is no more regulated by the FDA than it would be by the FAA or the Department of Motor Vehicles. For hundreds of patients, he and his team are providing a remarkable hope. They?ve brought lab-cultured medical stem cell therapies to the US. Finally.
Stem cells have been a focal point for hype and hope for years now. Besides healing horses and dogs, they have promising effects on diabetes, corneal blindness, even HIV. It?s pretty clear that they?re also the future of organ transplants. Just the news of a stem cell related development or patent will cause a biotech company?s stocks to soar. The FDA, which regulates all interstate drug sales and related clinical trials is not trying to keep Americans from these ?miraculous? cures, it?s simply trying to make sure they are safe first. Apparently, that?s taking too long. Medical tourism agencies are starting to cater to those seeking stem cell treatments. Whether or not they are ready for widespread medical use, stem cell therapies are in high demand, not just in the US but around the world. It?s no longer a question of when we will have access to these treatments, it?s a question of how.
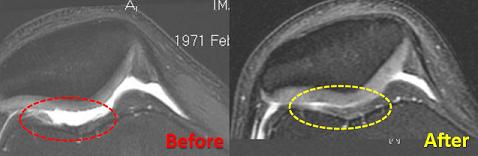
A severely damaged knee healed to a remarkable degree. Must be stem cells. RSI is offering adult stem cell therapies in the US. That’s an important first, but what will happen without FDA approval?
Patient?s interested in the Regenexx procedure face what seems to be a fairly standard experience for autologous stem cell transplants. It takes 20-40 minutes to extract the cells from hip bone marrow with limited anesthesia, and blood is also taken. Over a month RSI?s lab will isolate mesenchymal (multipotent) adult stem cells and multiply them until they have 1 to 10 million. Typically, a patient will receive an injection into the treated area once a month for three months. Positive results are sometimes seen quickly (in 1 to 3 months) but will hopefully develop within 6 to 9 months. Importantly, there?s no down time as a result of the procedure. Patients can leave the clinic and go home after each injection.? A round of Regenexx (extraction, cultivation,? and 3 injections) costs $7000-$8500. Those who produce exceptional numbers of stem cells can use subsequent injections (even in other parts of the body) for around $3500. Most insurances will not cover the treatment.
The fact that RSI isolates and cultures (multiplies) the cells is a big difference from other clinics that offer stem cell therapies. That process allows the lab to create enough mesenchymal stem cells? to really have an effect on the area in which they are injected. Many clinics around the world will take blood, marrow, or tissue and then spin out the stem cells in a centrifuge, injecting them back in on the same day. That style? of therapy could possibly be effective, but it is far less likely than with a dose of millions of multipotent stem cells. There are several doctors around the US that will provide such ?single-visit? stem cell therapies, but RSI is offers the lab cultured mesenchymal therapy in the US. Dr. Centeno has confirmed that he?s the only one, that he knows of, openly using this particular procedure in the US.
In the past, many have been very skeptical of stem cell treatment centers in other countries. First, let?s look at the success RSI is selling. Autologous transplants are offered in the hands, hips, knees, shoulders, back (non-spinal cord injury), ankles, and bone fractures. For each of these procedures you can find many ardent and exceptionally encouraging patient testimonials on their website, or their YouTube channel, along with a flood of supportive media.
Overall, RSI is claiming around 80% patient satisfaction according to its own surveys. That?s incredible, especially when you see some of their patients walking and running again on joints that have experienced years of chronic damage. It also seems Centeno and Schultz have the documented evidence to back up the claims for Regenexx?s success. RSI provides case studies for each of its treatments as well as published scientific research. According to my conversation with Centeno, RSI is currently working on a comprehensive statistical analysis of their more popular treatments so they can publish quantitative results in a peer review journal. In other words, they?ll soon publish the hard numbers ? X% of patients feel Y% better Z months after the procedure.
Importantly, RSI seems to be upfront with patients about the limits of their own technique. The website FAQ clearly states that not all results will be like the testimonials, and they even have a dedicated page explaining that stem cell therapies won?t work for everyone. Furthermore, RSI has published the largest study of risks and complications associated with stem cell treatments yet produced in the US (N=227). That paper demonstrates the very low harm associated with stem cell therapies ? much lower than the alternative surgery(published in Current Stem Cell Research & Therapy). Centeno claims that if we?re really worried that autologous stem cell therapies are going to hurt someone, this paper pretty much shows they won?t.
The concerns most people have with RSI are not medical, they?re political. Many applaud Centeno and Schultz for supplying the public with the cutting edge technology they demand, but worry about the manner in which it has been accomplished. Skirting FDA approval for a technique through the arguments they use opens the gate to a host of problems. If RSI can provide Regenexx because it is a doctor?s procedure not involved in interstate commerce, does that mean someone else can do the same for another treatment? What are the limits of such procedures? How does a patient know if a doctor?s therapy is safe, or effective, if it hasn?t undergone peer review and government inspection?
Doctors and surgeons are developing new procedures all the time. Surgeons will often create new devices for their own use in surgery, doctors routinely try out new dosing regimes, or therapies on their patients. This is part of the medical profession.
Still, it?s possible that even though RSI is doing what many other doctors routinely do (develop a new therapy for use in their own practice) that the federal government could try to bring them to court. The FDA seems to have taken the stance that all stem cells (whether used autologously or not) are drugs. As such, they would need FDA approval, and would likely only be developed by large pharmaceutical companies.
According to Lee Buckler of Cell Therapy Blog, Centeno?s already received a warning letter from the FDA. Centeno clarified that this is actually an ?untitled letter? which has no bearing on regulation. He pointed me to this explanation on untitled letters. RSI has faced concerns from the New York Department of Health, and went so far as to pursue a provisional license, even though they are no where near NY state. Clearly RSI is hoping to avoid bureaucratic problems or at least be very prepared for them if they do arise.? Perhaps with enough positive results they can avoid legal battles and even convince insurance companies to cover Regenexx.
Hopefully so. Just look as these results. They?re pretty damn amazing. If you accept the success rates, and the possibilities for long term healing?many people need this.
Centeno says he is working with others to provide the framework through which many more patients could receive mesenchymal stem cell therapies. He?s on the board of the International Cell Medicine Society (ICMS) which is working to track stem cell therapy patients through a registry, as well as certify stem cell clinics for practice. Through conferences and seminars, doctors are trained in IVF to work in fertility clinics. Centeno explained to me that a similar practice could instruct and track physicians interested in providing lab cultured autologous stem cell therapies. In other words, the technique used by RSI could become a regularly seen procedure in specialty clinics across the country. That may mean more patients could have access to stem cells soon.
FDA approval is slow, but it?s coming. Athersys has a patent for a stem cell derived drug, other companies have therapies in clinical trials. Those treatments will be here some day. In the meantime, RSI is filling in the gap. Their work may even catch on as a trend. If largely successful, insurance companies may pay for it and the federal government may end up grandfathering Regenexx in at some point. It could happen. What?s certain is that the public demand for stem cell therapies is real, growing, and seemingly justified. When that sort of pressure for a technology exists nobody can keep it down.
There is no conclusive understanding of what the long term effects of stem cells treatments will be. We do not know if a stem cell treatment will be effective 5-10 years after it is administered, and we know of no large study that has conclusively reviewed patients for cancer, or any long term side effect 5-10 years after a stem cell injection. Part of what ICMS is trying to do (reviewing clinics, tracking patients results over the long term) may yield a better understanding in the future.
[Sources: Regenerative Sciences Inc, ABC 7 News in Denver, Lee Buckler , RSI Blog, Current Stem Cell Research & Therapy]





